There are two distinct forms of cloning such as reproductive cloning (making copies of entire organisms) and therapeutic cloning (making copies of embryonic stem cells). With very rare exceptions, the vast majority of researchers and medical professionals are against the idea of reproductive cloning in humans. However, there is a growing popularity for the pursuit of therapeutic cloning since the promises of stem cell research are so enticing. As the result, some people think that both are unacceptable, others think both fine and some are in favour of one but not the other.
According to me, therapeutic cloning is fine whereas reproductive cloning is unacceptable.
Firstly, therapeutic cloning can be used to understand human biology. For example, scientists can do experiments based on therapeutic clones of human organs. In addition, they could grow to repair a serious burn, grow new heart muscle to repair an ailing heart, grow new kidney tissue to rebuild a failing kidney.
Secondly, reproductive cloning is unacceptable. The reason is that by cloning an organism, scientist just copy the organism. When there are lots of copies for an organism, genetic differences cannot be seen between those copies. In the long term, those organisms can cause genetic illnesses because of the fertilization between those clones. For this reasons, reproductive cloning shouldn't be used to protect the genetic diversity between the organisms whereas therapeutic cloning can be used for experiments and growing human organs for illnesses.
Gülşen Seçen
Bu Blogda Ara
1 Mayıs 2012 Salı
18 Mart 2012 Pazar
Do you think the advances in genetics and what we know about human genetics prove that humans are more similar to each other, or that humans are more different from each other?
According to me, everyting in the world is unique. Even though there are classification systems between the organisms, we all have common things through the creation of organisms. In the opposite, the smlla differences make us different from each other. For example; there are red, green, blue things arounds us. They have the same thing inside, even though they seem different from outside. I don't have an evidence for that. Baceuse this is how I explain the meaning of life. In order to response to the question, all humans are different as the individual people, however we are all same inside. The things that we create make us different. There is nothing that is created differently from another organism.
19 Şubat 2012 Pazar
Page 99-Pink TOK box
A karyotype is a way to find out if a baby has an atypical number of chromosomes. However, the anormal chromosomes cannot be fixed. For this reason, a karyotype is necessary to understand the situation before the birth of the baby. If there is a big possibility of atypical number of chromosomes for a baby and the family doesn't want a baby that has the illness, karyotype is necessary. There are two steps to understand if karyotype is necessary.
1. Does the family want the baby whether or not if the baby has the anormality?
If yes, the karyotype is not necessary.
2.If the answer for the first question is no, is there a big possibility of having anormal numbers of chromosomes for the baby?
If yes, the karyotype is necessary.
First question is about the family decision, and the second question is about the doctors predictions.
First question should be answered by the family absolutely taking informations from the doctors. Nobody has power to take the decision of aborting a baby without the decision of the family.
If I found out that my future child had a chromosomal anormaly that would make him or her very different from other children, I would not make him or her to have a life like that. Every parent want to make their children to have better lives. If my child would not able to breath without help, I would not make him or her to live.
1. Does the family want the baby whether or not if the baby has the anormality?
If yes, the karyotype is not necessary.
2.If the answer for the first question is no, is there a big possibility of having anormal numbers of chromosomes for the baby?
If yes, the karyotype is necessary.
First question is about the family decision, and the second question is about the doctors predictions.
First question should be answered by the family absolutely taking informations from the doctors. Nobody has power to take the decision of aborting a baby without the decision of the family.
If I found out that my future child had a chromosomal anormaly that would make him or her very different from other children, I would not make him or her to have a life like that. Every parent want to make their children to have better lives. If my child would not able to breath without help, I would not make him or her to live.
7 Şubat 2012 Salı
Malaria and the Red Cell
Malaria and the Red Cell
Natural Selection
Human defense systems and pathogens (organisms such as bacteria that produce disease) engage in constant stuggle, with the outcome being health or illness. Each year, for instance, millions of people battle the rhinoviruses that produce the common cold. The prize for the winners is a day at school or work. The penalty for the losers often is a miserable day at home. Fortunately, the losers survive and have a chance to redeem themselves when battle resumes the following year.Sometimes the stakes are higher, however. People and pathogens also engage in struggles where the prize is life itself. Each winter, along with the rhinovirus wars, humankind engages in battles with the influenza viruses. The influenza viruses are far more potent than are the rhinoviruses, however. Death from influenza is a well-know risk, particularly for the elderly and people whose defense systems are weakened by chronic illness. Fortunately, the elderly and infirm now have medical science as a powerful ally that bolsters their defenses with immunizations to prevent influenza attack or drugs that suppress the influenza virus once it has struck.
These pathogen wars take place in the context of constant changes in our physical characteristics. Our genetic essence resides in a molecule called DNA which is the master blueprint to our makeup. We receive half our DNA from each parent. However, a few spontaneous alterations occur in everyone's DNA so that we are not a perfect melding of our two parents. These changes in the DNA molecule are called "mutations". The overwhelming majority of mutations are minor and inconsequential. The individual with the mutation neither suffers an illness nor dies as a result. By the same token, the mutation imparts no survival advantage over other people. These are "neutral" mutations.
Rarely, mutations are detrimental to health. If the mutation is so severe that the person dies before procreation, the muation dies with them and is not passed into the next human generation. These are "negative" mutations. A mutation that severely impairs the body's defense system against bacterial infection, for instace would fall into this category.
Even less common are mutations that give the recepient an advantage over other people. Sometimes the advantage improves the ability to survive a potentially deadly illness. The affected individual can then pass his/her genes to the next generation more efficiently than other people because they are more likely to reach reproductive age. This increases the chance that the modified gene will survive into the first generation (that of the children) and from there move into the following generation (that of the grandchildren). This is a "positive" mutation.
Natural selection has to be considered in the context of "pre-modern" societies. Modern medicine has altered the balance of nature and often allows us to rescue people who otherwise would die of their condition. A case in point is juvenile diabetes. Untreated, the disorder often is fatal in childhood. Modern medicine allows most people with juvenile diabetes to live essentially normal lifes. Natural selection consequently is no longer an issue for most people with juvenile diabetes. The same is true for many other hereditary disorders. When we examine traits in the human population that rose to high frequency through natural selection, we are in effect peering through the looking glass at our distant past.
A common misconception is that natural selection somehow produces a desirable change: "giraffes grew long necks in order to reach leaves high in trees." This is notthe way in which natural selection works, however. Natural selection does not promote or produce a change in an organism. Rather, a change occurs because of spontaneous alterations or mutations in the DNA genetic code. Changes in the genetic code can alter the physical characteristics of the organism. If the new trait gives the organism a survival or reproductive advantage over its fellows, the new trait will be represented in the second generation more frequently than it was in the first generation. This is the natural process by which advantageous characteristics are selected.
Malaria
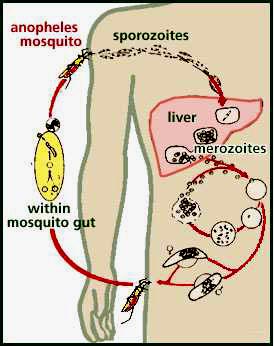 |
| Figure 1. The life cycle of the malaria parasite. From "Mosquito Bites";http://whyfiles.org/016skeeter/malaria2.html |
The plasmodium parasite that causes malaria is transmitted from mosquitos to men. The parasites spend part of their life cycle in the mosquito and part of it in the human host (Figure 1). The infective plasmodial sporozoites enter the bloodstream from the saliva of the feeding female anopheles mosquito. The Kupfer cells of the liver clear the sporozoites from the blood stream and kill many of the organisms. A fraction of the sporozoites escape destruction however, and penetrate the hepatocytes where they take up residence.
The parasites within the hepatocytes transform into a new entity called schizonts. The nuclear genetic material in the schizonts replicates to the point that the hepatocytes are totally filled with new forms called merozoites. A single schizont can produce thousands of merozoites. Erumpent hepatocytes release the merozoites into the bloodstream where they invade circulating red cells. After penetrating the red cells the merozoites assume a ring form calledtrophozoites. These organisms consume hemoglobin in erythrocytes and enlarge until they fill the cell completely. During their growth, the trophozoites metamorph into schizonts and produce new merozoites inside the red cells. The red cells subsequently lyse and release merozoites that can penetrate new red cells and restart the pernicious process.
Some of the trophozoites in the red cells take a different developmental pathway and form gametocytes. Gametocytes are the sexual form of the parasite and do no lyse the red cells. A mosquito taking a blood meal from a person whose red cells contain gametocytes acquires the malarial parasite. The sexual reproduction cycyle then begins in the mosquito. The mosquito subsequently transmits the parasite when it attacks another human host.
Malaria Defenses
The complex nature of the malaria parasite life cycle in the human host presents several points at which the organism could be targeted for destruction. The sporozoites injected into the blood stream with the initial mosquito bite are attacked there by components of the immune system. These include antibodies, lymphocytes called "natural killer cells" as well as lymphocytes that attack the malarial parasites because of prior exposure to the organisms (conditioned lymphocytes).Host immunity is crucial to survival of people infected with the malaria parasite. This is particularly true with respect to the nocuous falciparum parasite. The immune system works best when it has been primed against the invader. Children who suffer their first or second bout of malaria have not developed the immune response needed to provide adequate defense against the parasite. This explains in part the high mortality seen in children infected with P. falciparum. Vaccines are a common way of achieving host immunity prior to pathogen exposure. Polio immunization is a well-known example. Unfortunately, the malarial parasite constantly changes its immune makeup, thereby frustrating efforts to produce an effective vaccine.
The intrahepatic phase of malarial parasite growth presents another potential point at which to attack the organism. No mutation in the structure or function of hepatic cells that kills the malarial parasite or retards its growth is known.
The last point at which life cycle of the malarial parasite can be frustrated in humans is at the phase of red cell invasion and multiplication. Red cells are constantly created and destroyed as part of their life cycle. A mutation that somehow destroys both the infected red cells and the parasite could therefore eliminate the malaria parasite. The destroyed infected cells would be replaced by new, healthy cells.
| Cell Component | ||
| Membrane | ||
| Hemoglobin | ||
| Red cell enzymes |
Table 1 lists some of the red cell defenses against malaria that have arisen by natural selection. The mechanisms by which some of these alterations thwart the malaria parasite are well-characterized while others are not.
At the red cell membrane, the Duffy antigen is the molecule used by the parasite P. vivax to enter the red cell. The high association of Duffy antigen null rell cells in some groups of people with sickle cell trait suggested that the Duffy antigen might provide some protection against malaria (Gelpi and King, 1976). Later investigations showed the Duffy antigen to be the receptor by which the merozoites of P. vivax enter red cells. People who lack the Duffy antigen (FY*O allele) are resistant to P. vivax (Hamblin and Di Rienzo, 2000). The Duffy null phenotype is most common in people whose ancestors derive from regions in Africa where vivax malaria is endemic.
Sickle hemoglobin provides the best example of a change in the hemoglobin molecule that impairs malaria growth and development. The initial hints of a relationship between the two came with the realization that the geographical distribution of the gene for hemoglobin S and the distribution of malaria in Africa virtually overlap. A further hint came with the observation that peoples indigenous to the highland regions of the continent did not display the high expression of the sickle hemoglobin gene like their lowland neighbors in the malaria belts. Malaria does not occur in the cooler, drier climates of the highlands in the tropical and subtropical regions of the world. Neither does the gene for sickle hemoglobin.
Sickle trait provides a survival advantage over people with normal hemoglobin in regions where malaria is endemic. Sickle cell trait provides neither absolute protection nor invulnerability to the disease. Rather, people (and particularly children) infected with P. falciparum are more likely to survive the acute illness if they have sickle cell trait. When these people with sickle cell trait procreate, both the gene for normal hemoglobin and that for sickle hemoglobin are transmitted into the next generation.
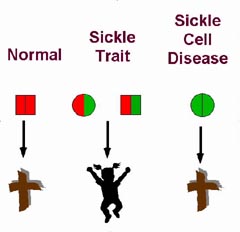 |
| Figure 2. Schematic representation of the effect of the sickle cell hemoglobin gene on survival in endemic malarial areas. People with normal hemoglobin (left of the diagram) are susceptible to death from malaria. People with sickle cell disease (right of the diagram) are susceptible to death from the complications of sickle cell disease. People with sickle cell trait, who have one gene for hemoglobin A and one gene for hemoglobin S, have a greater chance of surviving malaria and do not suffer adverse consequences from the hemoglobin S gene. |
Figure 2 is a schematic of the natural selection that occurs with the gene for sickle hemoglobin in areas endemic forP. falciparum malaria. The left-hand side of the panel shows the situation in people with two genes encoding normal hemoglobin A (designated by red). These people have a significant chance of dying of acute malarial infection in childhood. In contrast, people with two genes for sickle hemoglobin (shown in green) are likely to succumb to sickle cell disease at an early age, as shown in the right-hand side of the figure.
In the center are people with sickle cell trait who possess one gene for normal hemoglobin and one gene for sickle hemoglobin. These children are more likely to survive their initial acute malarial attacks than are people with two genes for normal hemoglobin. Also, they suffer none of the morbidity and mortality of sickle cell disease. Therefore, the people with sickle cell trait are more likely to reach reproductive age and pass their genes on to the next generation (Ringelhann, et al., 1976).
The genetic selective scenario in which a heterozygote for two alleles of a gene has an advantage over either of the homozyous states is called "balanced polymorphism". A key concept to keep in mind is that the selection is for sickle cell trait. A common misstatement is that malaria selects for sickle cell disease. This is not true. A person with sickle cell disease is at an extreme survival disadvantage because of the ravages of the disease process. This means that a negative selection exists for sickle cell disease. Sickle cell trait is the genetic condition selected for in regions of endemic malaria. Sickle cell disease is a necessary consequence of the existence of the trait condition because of the genetics of reproduction.
The precise mechanism by which sickle cell trait imparts resistance to malaria is unknown. A number of factors likely are involved and contribute in varying degrees to the defense against malaria.
Red cells from people with sickle trait do not sickle to any significant degree at normal venous oxygen tension. Very low oxygen tensions will cause the cells to sickle, however. For example, extreme exercise at high altitude increases the number of sickled erythrocytes in venous blood samples from people with sickle cell trait (Martin, et al., 1989). Sickle trait red cells infected with the P. falciparum parasite deform, presumably because the parasite reduces the oxygen tension within the erythrocytes to very low levels as it carries out its metabolism. Deformation of sickle trait erythrocytes would mark these cells as abnormal and target them for destruction by phagocytes( Luzzatto, et al., 1970).
Experiments carried out in vitro with sickle trait red cells showed that under low oxygen tension, cells infected with P. falciparum parasites sickle much more readily than do uninfected cells (Roth Jr., et al., 1978). Since sickle cells are removed from the circulation and destroyed in the reticuloendothelial system, selective sickling of infected sickle trait red cells would reduce the parasite burden in people with sickle trait. These people would be more likely to survive acute malarial infections.
Other investigations suggest that malaria parasites could be damaged or killed directly in sickle trait red cells. P. falciparum parasites cultured in sickle trait red cells died when the cells were incubated at low oxygen tension (Friedman, 1978). In contrast, parasite health and growth were unimpeded in cells maintained at normal atmospheric oxygen tensions. The sickling process that occurs at low oxygen tensions was presumed to harm the parasite in some fashion. Ultrastructural studies showed extensive vacuole formation in P. falciparum parasites inhabiting sickle trait red cells that were incubated at low oxygen tension, suggesting metabolic damage to the parasites (Friedman, 1979). Prolonged states of hypoxia are not physiological, raising questions about degree to which these data can be extrapolated to human beings. However, they do suggest mechanisms by which sickle hemoglobin at the concentrations seen with sickle cell trait red cells could impair parasite proliferation.
Other investigations suggest that oxygen radical formation in sickle trait erythrocytes retards growth and even kills the P. falciparum parasite (Anastasi, 1984). Sickle trait red cells produce higher levels of the superoxide anion (O2-) and hydrogen peroxide (H2O2) than do normal erythrocytes. Each compound is toxic to a number of pathogens, including malarial parasites. Homozygous hemoglobin S red cells produce membrane associated hemin secondary to repeated formation of sickle hemoglobin polymers. This membrane-associated hemin can oxidize membrane lipids and proteins (Rank, et al., 1985). Sickle trait red cells normally produce little in the way of such products. If the infected sickle trait red cells form sickle polymer due to the low oxygen tension produced by parasite metabolism, the cells might generate enough hemin to damage the parasites (Orjih, et al., 1985).
The immune system is key to weathering attacks by P. falciparum. Maternal antibodies passed to newborns prior to birth provide some protection from malaria for the first several months of life. Thereafter, the onus is on the toddler's immune system to provide the needed defense. Epidemiological studies performed in regions with endemic malaria show that antibody titers to P. falciparum are lower in children with sickle cell trait than in children with genes only for hemoglobin A (Cornille-Brogger, et al., 1979). The investigators speculated that lower levels of immune activation might reflect a lower parasite burden in children with sickle cell trait due to clearance of the infected red cells. Analysis of people with sickle cell trait and people homozygous for hemoglobin A in the regions with endemic malaria in fact show a lower mean parasite burden in people with sickle cell trait relative to hemoglobin A homozygotes (Fleming, et al., 1979). In contrast, children with sickle cell disease have a high fatality rate, with acute malarial infections being a chief cause of death (Fleming, 1989).
Hemoglobin C is also believed to protect against malaria, although data on this point were not conclusive until recently. Hemoglobin C lacks the in vitro antimalarial activity of hemoglobin S. Some epidemiological studies found no evidence for protection against malaria in people with either homozygous or heterozygous hemoglobin C (Willcox, et al., 1983). The relatively small number of patients with hemoglobin C in these studies left the conclusions open to question, however.
The issue was finally settled in an investigation that included more than 4,000 subjects (Modiano, et al., 2001). Hemoglobin C heterozygotes had significantly fewer episodes of P. falciparum malaria than did controls with only hemoglobin A. The risk of malaria was lower still in subjects who were homozygous for hemoglobin C. Homozygous hemoglobin C produces a mild hemolytic anemia and splenomegaly. The much milder phenotype of the condition relative to homozygous hemoglobin S led the investigators to speculate that without medical intervention for malaria, hemoglobin C would replace hemoglobin S the over the next few thousand years as the dominant "antimalarial" hemoglobin in West Africa.
The thalassemias also reached levels of expression in human populations by protecting against malaria. The inbalance in globin chain production characteristic of thalassemia produces membrane oxidation by hemichromes and other molecules that generate reactive oxygen species (Grinberg, et al., 1995;Sorensen, et al., 1990). Reactive oxygen species also injure and kill malaria parasites (Clark, et al., 1989).
In vitro malaria toxicity of thalassemic red cells is most easily seen in cells containing hemoglobin H (ß-globin tetramers) (Ifediba, et al., 1985; Yathavong, et al., 1988). Hemoglobin H occurs most often in people with three-gene deletion alpha-thalassemia (Zhu, et al., 1993). The compound heterozygous condition of two-gene deletion alpha thalassemia and hemoglobin Constant Spring also produces erythrocytes that contain hemoglobin H (Derry, et al., 1988). Two gene deletion alpha thalassemia also protects the host from malaria, however. The process is difficult to demonstrate with in vitro cultures of malaria parasites. Alpha thalassemia may protect against malaria in part by altering the immunue response to parasitized red cells (Luzzi, et al., 1991) In any event, epidemiological studies show clear evidence of protection provided by two-gene deletion alpha thalassemia (Flint, et al., 1986; Modiano, et al., 1991).
One of the key reasons for the high fatality rate in P. falciparum malaria is the occurence of so-called cerebral malaria. Patients become confused, disoriented and often lapse into a terminal coma. Clumps of malaria-infested red cells adhere to the endothelium and occlude the microcirculation of the brain with deadly consequences. The P. falciparum parasite alters the characteristics of the red cell membrane, making them more "sticky". Clusters of parasitized red cells exceed the size of the capillary circulation blocking blood flow and producing cerebral hypoxia. Thalassemic erythrocytes adhere to parasitized red cells much less readily than do their normal counterparts (Carlson, et al., 1994). This alteration would lessen the chance of developing cerebral malaria.
The rise to high frequency of alleles that produce red cells deficient in glucose-6-phosphate dehydrogenase activity is one of the most dramatic examples of the selective pressure of malaria on humankind (Ruwende, et al., 1995; Tishkoff, et al., 2001). Reactive oxygen species are formed continually as erythrocytes take up oxygen from the lungs and release it to the preriperal tissues. As noted above, malaria parasites are easily damaged by these reactive oxygen species (Friedman, 1979). Glucose-6-phosphate dehydrogenase prevents oxidation of the heme group. In its absence, hemichromes and other species that generate reactive oxygen species accumulate in erythrocytes (Janney, et al., 1986). P. falciparum grow poorly in erythrocytes that are deficient in G-6-PD (Roth JR, et al., 1983). Malaria continues to battle back in this struggle, however. The advent of P. falciparum parasites that produce their own G-6-PD provides ample evidence of the continuing moves and counter-moves in the battle between man and malaria (Usanga, et al, 1985).
Malaria
| Home > People > News > Malaria and sickle cell trait | |
While it has been known for some time that sickle cell trait offers protection against malaria, the mechanisms have never been clear. Now, a study - led by Dr Tom Williams of the Kenya Medical Research Institute/Wellcome Trust Research Programme in Kilifi, Kenya - has discovered an unexpected link with immunity. Children with sickle cell trait have shown enhanced immunity to the malaria parasite, with the level of immunity increasing with age. Sickle cell trait occurs when someone inherits a normal haemoglobin gene from one parent (HbA) and a sickle haemoglobin gene (HbS) from the other (resulting in HbAS). While children with sickle cell trait do not usually display symptoms, children who inherit a double dose of the sickle gene suffer from sickle cell disease, which can cause chronic ill health and early death. Further research will be needed to find out why the trait leads to increasing levels of immunity. One possibility, suggested by Dr Williams, is that a malaria infection persists in the body for longer, so allowing the immune system time to build up a better defence. The blood cell abnormality, sickle cell trait, gives an increasing amount of protection against malaria as young children grow during their first ten years of life, new research has revealed. Between the ages of two and ten immunity to the disease, which kills up to two million people a year, rises rapidly, a Wellcome Trust-funded study has found. The project involved over 1000 people living on the coast of Kenya, where malaria is rife. Those taking part were aged from three months to 84 years, although most were under ten. The research, which is reported by the Public Library of Science today (31 May 2005), was carried out over six years, and compared the incidence of mild malaria in those with sickle cell trait and those without. The trait causes blood cells to deform – into the shape of a scythe – but does not cause sickle cell disease, which, in its worst form, can cause anaemia, lung problems and strokes. People with the trait, which is inherited, lead normal lives. The study, which was led by Dr Tom Williams of the Kenya Medical Research Institute/Wellcome Trust Research Programme in Kilifi, found that the increased protection from sickle cell trait (HbAS) rose from 20 per cent in the first two years of life to over 50 per cent by the age of ten. Dr Williams said: "It has been known for some time that sickle cell trait offers this protection, but the accelerated level of immunity in the first years of life has not been revealed before. "There are several possible reasons why this happens and further research will be required before we find the full answer. "But one explanation may be that HbAS causes the malaria infection to stay in the body a long time, so allowing the immune system time to build up a proper defence. "Our research concentrated on mild malaria, which causes fever, but does not kill. We do not know if our findings can be applied to severe forms of malaria, which can lead to death." ReferencesWilliams TN et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005 May;2(5):e128. Epub 2005 May 31. Abstract; full text LinksPage of 2 19/7/06 [WTD023878] Malaria and sickle cell trait.doc |
|
What is the relationship between the sickle cell anemia and the malaria?
Sickle cell anemia is a genetic diseases that causes red blood cells to be shaped like sickles while normal red blood cells are shaped more like a cylinder. Malaria works by going into the red blood cells until they burst. Because of the strange shape of the red blood cells in people who have sickle cell anemia, malaria can't enter in the cells that have the sickle trait, but it can enter in the others. This way, people with sickle cell wouldn't be as adversely effected then those without it. Having one copy of chromosome mutation in the hemoglobin gene will be protective against malaria and while having two copies of mutation in the hemoglobin gene will cause the individual to suffer from sickle cell anemia. Actually there is no causation between two diseases. Sickle cell anemia is a natural barrier in front of the malaria. Normally diseases are harmful in all ways. However sickle cell anemia prevents malaria as an advantageous situation for this correlation. A sickle cell anemia carrier is in a advantageous situation while fighting with malaria. Through the natural selection, people might become resistant to the malaria.
18 Aralık 2011 Pazar
Protecting Nature
The Nature Conservancy is taking on the tough issues facing conservation today — from climate change to coral reefs, to energy development in a growing world. We are applying high level strategies to our conservation work around the world in the following areas:
Conservation Action
The Conservancy works with government officials and partners to support public policies that protect our lands and waters so the next generations of Americans can build secure and rewarding lives.
Climate Change
Working for solutions that will reduce emissions to levels compatible with a healthy planet, preserve forests and help nature adapt to global warming.
Rainforests
The Nature Conservancy is working around the world in places like Costa Rica's Osa Peninsula to protect rainforests, engaging local and indigenous communities in creative solutions that balance the needs of people with nature.
Coral Reefs
Scientists estimate that unless we take immediate action, we could lose up to 70 percent of coral reefs by 2050.The Nature Conservancy is dedicated to protecting these vital ecosystems and all the corals, fish and people that depend on them.
Migratory Birds
Birds are a priceless part of our heritage. They are beautiful, they are economically important — and birds reflect the health of our environment. Preserving and protecting bird habitat has always been a core part of the Conservancy's mission.
Land Conservation
Building on our tradition of protecting lands and waters, we are working to balance growing development needs with those of nature; build relationships with communities, companies and governments; and increase funding for large-scale conservation projects.
People and Conservation
Protecting nature isn’t about putting up fences around pristine places to keep people out. We’re about protecting the places and resources we depend on for the benefit of all species — plants, animals and people.
How We Work
Our Science
The Conservancy is a world leader in cutting-edge conservation science p with 550 staff scientists around the globe.
Conservation by Design
For more than a decade, The Nature Conservancy’s work has been guided by a framework we call Conservation by Design — a systematic approach that determines where to work, what to conserve, what strategies we should use and how effective we have been.
Development by Design
Development by Design balances the needs of planned development — such as oil and gas, mining, and infrastructure — with those of nature conservation. The approach supports decision-making on how best to avoid conflict, maintain biodiversity and determine effective mitigation responses.
Working with Indigenous People
A "Commitment to People, Communities and Cultures" is a central part of The Nature Conservancy’s core values. We are committed to local, on the ground involvement; we treat our partners with fairness and honesty; and we work collaboratively to develop practical conservation solutions.
Pursuing Diversity
The Nature Conservancy has a strong and abiding commitment to diversity in its workforce and in the people and groups with which it works. We recognize the Conservancy's mission is best advanced by the leadership and contributions of men and women of diverse backgrounds, beliefs and cultures.
Working with Companies
For decades, The Nature Conservancy has recognized that the private sector has an important role to play in advancing our conservation mission. We are applying our science, reach, expertise in conservation planning, and on-the-ground experience to help businesses make better decisions, understand the value of nature, and protect it.
Kaydol:
Yorumlar (Atom)

